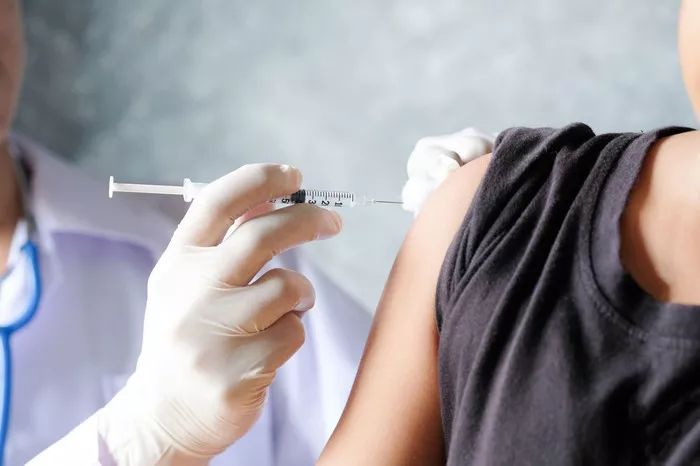
Epinephrine auto-injectors (EAIs) like EpiPen have long been the gold standard for treating anaphylaxis. However, their reliance on needles can cause hesitation in use, especially among children and needle-phobic individuals. Recent advancements have introduced a promising needle-free alternative: an epinephrine nasal spray.
Researchers at a leading pharmaceutical company have completed Phase III clinical trials for an intranasal epinephrine delivery system. The study, published in The Journal of Allergy and Clinical Immunology, demonstrated that the nasal spray achieved comparable plasma epinephrine levels to traditional intramuscular injections within minutes. The spray’s design allows for easy administration, making it particularly useful for young children and individuals who may struggle with auto-injectors.
One of the key advantages of this innovation is its stability. Unlike liquid epinephrine in auto-injectors, which can degrade if exposed to extreme temperatures, the nasal formulation remains effective under a wider range of storage conditions. This is particularly beneficial for patients in regions with limited access to refrigeration.
Despite these promising results, regulatory approval is still pending. The FDA has requested additional data on long-term safety and real-world usability. If approved, this could revolutionize anaphylaxis management, reducing barriers to timely treatment and potentially saving more lives.
Experts caution, however, that while this development is exciting, current guidelines still recommend carrying an EAI until the nasal spray is fully approved and widely available. Patients should consult their allergists before making any changes to their emergency treatment plans.
You Might Be Interested In:
- The Hidden Economic Burden of Hay Fever: Lost Productivity and Healthcare Costs
- How to Help Your Baby with Seasonal Allergies?
- Breakthrough in Immunotherapy Offers Hope for Severe Seasonal Asthma Sufferers